Catalysts Speed It Up

A catalyst is like adding a bit of magic to a reaction.
Reactions need a certain amount of energy in order to happen. If they
don't have it, oh well, the reaction probably can't happen. A catalyst
lowers the amount of energy needed so that a reaction can happen more
easily. A catalyst is about energy. It doesn't have to be another moelcule. If you fill a room with hydrogen gas (H 2) and oxygen gas (O 2),
very little will happen. If you light a match in that room (or just
produce a spark), most of the hydrogen and oxygen will combine to create
water molecules (H 2O). It is an explosive reaction.
The energy needed to make a reaction happen is called the activation energy.
As everything moves around, energy is needed. The energy that a
reaction needs is usually in the form of heat. When a catalyst is added,
something special happens. Maybe a molecule shift
...
Read more »
|
Equilibrium Basics

Equilibrium is a pretty easy topic - big name, but easy idea. First, when you have a system made up of a bunch of molecules, those molecules sometimes combine. That's the idea of a chemical reaction.
Second, a chemical reaction sometimes starts at one point and moves to
another. Now imagine the reaction finished and you have a pile of new
chemicals. Guess what? Some of those chemicals want to go through a
reverse chemical reaction and become the original molecules again. We
don't know why. Sometimes they just do.
Put those two ideas together and you have equilibrium:
1. Two reactants combine to make a product.
2. Products like to break apart and turn back into the reactants.
There is a point where those two reactions happen and you can't tell
that any reactions are happening. That's the point when the reaction
looks like it is finished. In reality, some of the molecules are turning
into products and some are turning back into reactants. You need to
imagine that you're as small as a molecule and you're
...
Read more »
|
Rate of Reaction
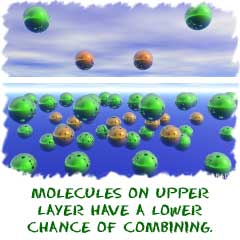
The rate of a reaction is the speed at which a reaction happens. If a reaction has a low rate, that means the molecules
combine at a slower speed than a reaction with a high rate. Some
reactions take hundreds, maybe even thousands, of years while others can
happen in less than one second. The rate of reaction depends on the
type of molecules that are combining. If you want to think of a very
slow reaction, think about how long it took dinosaur bones to become
fossils through breakdown. You can thank chemical processes in bacteria
for most of those dinosaur bones in the museum.
There is another big idea for rates of reaction called collision theory. The collision theory says that as more collisions in a system occur, there will be more combinations
of molecules bouncing into each other. If there are a higher number of
collisions in a system, more combinations of molecules can occur. The
reaction will go faster and the rate of that reaction will be higher.
Even though they are both liquids, think about how slowly molecules move
in honey whe
...
Read more »
|
Chemical Reactions
Let's start with the idea of a reaction. In chemistry, a reaction happens when two or more molecules interact and the molecules
change. That's it. What molecules are they? How do they interact? What
happens? The possibilities are infinite. When you are trying to
understand reactions, imagine that you are working with the atoms.
Imagine the building blocks are right in front of you on the table,
instead of billions of reactions in your beaker. Sometimes we do this
using our chemistry toys to help us visualize the movement of the atoms.
There are a few key points you should know about chemical reactions:
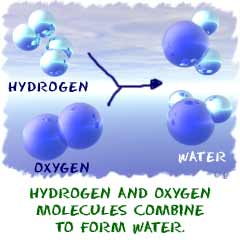 1.
1. A chemical change must occur. You
start with one compound and turn it into another. That's an example of a
chemical change. A steel garbage can rusting is a chemical reaction.
That rusting happens because the iron (Fe) in the metal combines with
oxygen (O 2) in the atmosphere. When a refrigerator or air
conditioner cools the air, there is no reaction between the air
molecules. The change in temperature is a physical change.
...
Read more »
|
Transitioning

Let's start off by telling you that there are a lot of elements that are considered transition metals. Which metals are the transition metals?
21 (Scandium) through 29 (Copper)
39 (Yttrium) through 47 (Silver)
57 (Lanthanum) through 79 (Gold)
89 (Actinium) and all higher numbers.
What Makes Them So Special?
It all has to do with their shells/orbitals. We like introducing students to the first eighteen elements, because they are easier to explain. Transition metals are good examples of advanced shell and orbital ideas. They have a lot of electrons
and distribute them in different ways. You will usually find that
transition metals are shiny, too. Not all of them, but we are sure
you've seen pictures of silver (Ag), gold (Au), and platinum (Pt).

Transition metals are able to put more than eight electrons in the shell
that
...
Read more »
|
Metal Basics

We wanted to give you a big overview of metals before
we get into details about specific families. Almost 75% of all elements
are classified as metals. They are not all like silver (Ag), gold (Au),
or platinum (Pt). Those are the very cool and shiny ones. There are
other metals like potassium (K) and iridium (Ir) that you might not
think about right away.
Many Kinds of Metals
How many kinds of metals are there? So many. Don't even try to memorize
them all. Just remember the ones you might need in class. Here's a quick
list: Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth
Metals, Rare Metals, Rare-Earth Metals, and Transition Metals.
Remember, that's the easy list. Lucky for you, the periodic table is excellent at organizing elements, and you will find each of these groups in specific areas of the periodic table.
How Do You Identify a Metal?

What are the characteristics of metals? We've got four
...
Read more »
|
The Noble Gases

We love the noble gases. Some scientists used to call
them the inert gases. It didn't really work because there are a few
other gases that are basically inert but not noble gases. Nitrogen (N 2)
might be considered an inert gas, but it is not a noble gas. The noble
gases are another family of elements, and all of them are located in the
far right column of the periodic table.
For all of you budding chemists, the far right is also known as Group
Zero (Group 0) or Group Eighteen (Group XVIII). This family has the
happiest elements of all.
Why Are They Happy?
Using the Bohr description of electron shells, happy atoms have full shells. All of the noble gases have full outer shells with eight electrons. Oh, wait! That's not totally correct. At the top of the noble gases is little helium
(He), with a shell that is full with only two electrons. The fact that
their outer shells are full means they are quite happy and don't need to
react with other elements. In fact
...
Read more »
|
Elements as Building Blocks
As you probably saw, the periodic table is organized like a big grid. The elements
are placed in specific locations because of the way they look and act.
If you have ever looked at a grid, you know that there are rows (left to
right) and columns (up and down). The periodic table has rows and
columns, and they each mean something different.
You've got Your Periods...

Even though they skip some squares in between, all of the rows go left
to right. When you look at a periodic table, each of the rows is
considered to be a different period (Get it? Like
PERIODic table.). In the periodic table, elements have something in
common if they are in the same row. All of the elements in a period have
the same number of atomic orbitals. Every element in the top row (the first period) has one orbital for its electrons.
All of the elements in the second row (the second period) have two
orbitals for their electrons. It goes down the periodic table like that.
At this time, the maximum number of electron orbitals or electron
shells for any el
...
Read more »
|
Compound Basics
Compounds are groups of two or more elements that are bonded together. You have also seen us use the word molecule. Molecule
is the general term used to describe atoms connected by chemical bonds.
Every combination of atoms is a molecule. Compounds happen with atoms
from different elements. So, all compounds are molecules, because they
have bonds between the atoms, like in water (H 2O). However, not all molecules are compounds because sometimes the atoms are of the same element. Hydrogen gas (H 2) is a good example of a molecule that is not a compound. There are two main types of chemical bond that hold atoms together: covalent and e lectrovalent/ionic bonds. Covalent compounds happen when the atoms share the electrons, and ionic compounds happen when electrons are donated from one atom to another.
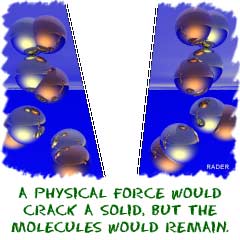
We talked about compounds and molecules in the
...
Read more »
|
Neutron Madness

We have already learned that ions are atoms that are either missing or have extra electrons. Let's say an atom is missing a neutron or has an extra neutron. That type of atom is called an isotope.
An atom is still the same element if it is missing an electron. The
same goes for isotopes. They are still the same element. They are just a
little different from every other atom of the same element.
For example, there are a lot of carbon
(C) atoms in the Universe. The normal ones are carbon-12. Those atoms
have 6 neutrons. There are a few straggler atoms that don't have 6.
Those odd ones may have 7 or even 8 neutrons. As you learn more about
chemistry, you will probably hear about carbon-14. Carbon-14 actually has 8 neutrons (2 extra). C-14 is considered an isotope of the element carbon.
...
Read more »
|
Looking at Ions

We've talked about ions before. Now it's time to get down to basics. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. A normal atom has a neutral
charge with equal numbers of positive and negative particles. That
means an atom with a neutral charge is one where the number of electrons
is equal to the atomic number. Ions are atoms with extra electrons or
missing electrons. When you are missing an electron or two, you have a
positive charge. When you have an extra electron or two, you have a negative charge.

What do you do if you are a sodium (Na) atom? You have eleven electrons — one too many to have an entire <
...
Read more »
|
Atoms Are Building Blocks
Atoms are the basis of chemistry. They are the basis for everything in the Universe. You should start by remembering that matter is composed of atoms. Atoms and the study of atoms are a world unto themselves. We're going to cover basics like atomic structure and bonding between atoms. As you learn more, you can move to the biochemistry pages and see how atoms form compounds that help the biological world survive.
Smaller Than Atoms?

Are there pieces of matter that are smaller than atoms? Sure there are.
You'll soon be learning that atoms are composed of pieces like electrons, protons, and neutrons.
But guess what? There are even smaller particles moving around in
atoms. These super-small particles can be found inside the protons and
neutrons. Scientists have many names for those
...
Read more »
|
Atomic Structure
The text provides a
historical perspective of how the internal structure of the atom
was discovered. It is certainly one of the most important
scientific discoveries of this century, and I recommend that you
read through it. However, we will begin our discussion of the
atom from the modern day perspective.
All atoms are made from
three subatomic particles
� Protons, neutron & electrons.
These particles have the
following properties:
|
|